Indefinite Shape And Definite Volume
3.3: Classifying Matter According to Its State—Solid, Liquid, and Gas
- Page ID
- 47454
- To describe the solid, liquid and gas phases.
Water can take many forms. At depression temperatures (below \(0^\text{o} \text{C}\)), it is a solid. When at "normal" temperatures (between \(0^\text{o} \text{C}\) and \(100^\text{o} \text{C}\)), it is a liquid. While at temperatures above \(100^\text{o} \text{C}\), water is a gas (steam). The state that water is in depends upon the temperature. Each state has its own unique prepare of physical backdrop. Matter typically exists in one of three states: solid, liquid, or gas.



The country that a given substance exhibits is also a physical property. Some substances exist as gases at room temperature (oxygen and carbon dioxide), while others, like h2o and mercury metal, be equally liquids. Well-nigh metals be as solids at room temperature. All substances can be in any of these three states. Figure \(\PageIndex{ii}\) shows the differences amongst solids, liquids, and gases at the molecular level. A solid has definite volume and shape, a liquid has a definite book only no definite shape, and a gas has neither a definite volume nor shape.
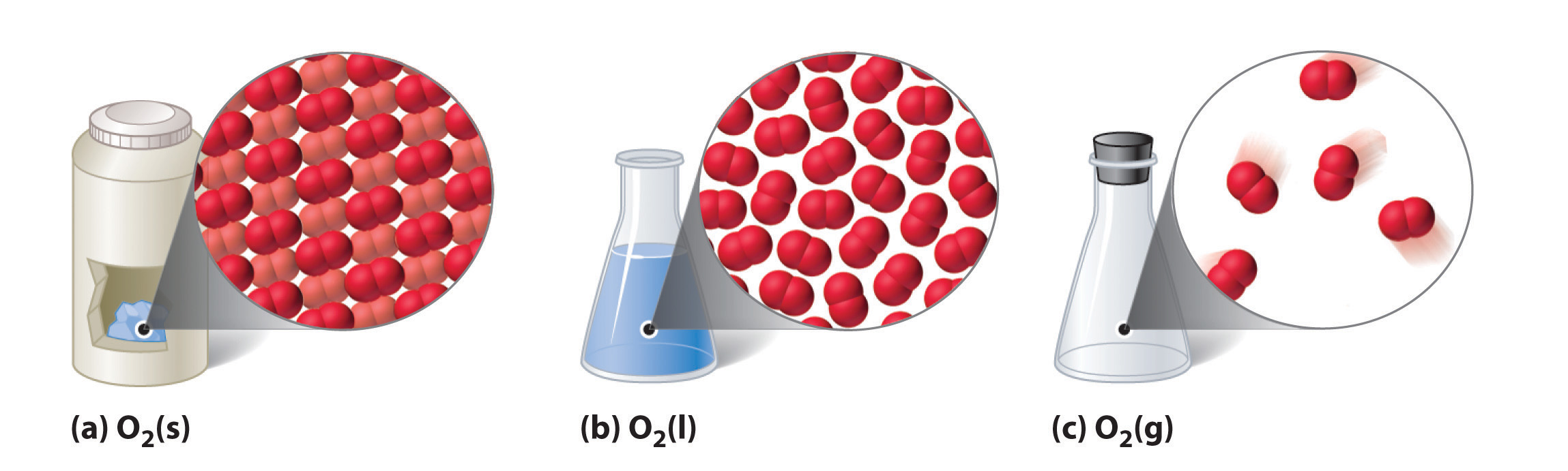
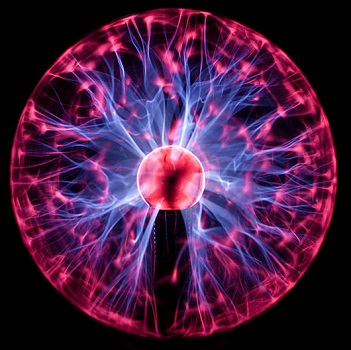
Technically speaking, a fourth state of matter called plasma exists, merely information technology does not naturally occur on earth, and then we will omit it from our written report here.
.jpg?revision=1&size=bestfit&width=197&height=196)
A plasma earth operating in a darkened room. (CC By-SA 3.0; Chocolateoak).
Solids
In the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is non enough thermal free energy to overcome the intermolecular interactions between the particles. Equally a result, solids have a definite shape and volume. Virtually solids are hard, only some (like waxes) are relatively soft. Many solids equanimous of ions tin can too exist quite brittle.
Solids are defined by the following characteristics:
- Definite shape (rigid)
- Definite book
- Particles vibrate around stock-still axes
If we were to cool liquid mercury to its freezing point of \(-39^\text{o} \text{C}\), and under the right pressure level conditions, we would notice all of the liquid particles would become into the solid country. Mercury tin be solidified when its temperature is brought to its freezing bespeak. Still, when returned to room temperature weather condition, mercury does not be in solid state for long, and returns back to its more than common liquid form.
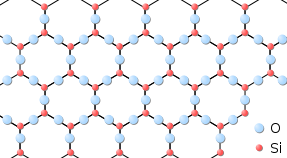
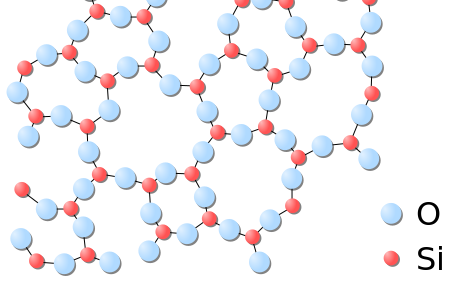
Solids usually take their constituent particles arranged in a regular, three-dimensional array of alternating positive and negative ions chosen a crystal. The outcome of this regular arrangement of particles is sometimes visible macroscopically, as shown in Effigy \(\PageIndex{3}\). Some solids, especially those composed of big molecules, cannot hands organize their particles in such regular crystals and exist equally amorphous (literally, "without course") solids. Glass is one example of an amorphous solid.
Liquids
If the particles of a substance have enough free energy to partially overcome intermolecular interactions, so the particles can movement well-nigh each other while remaining in contact. This describes the liquid state. In a liquid, the particles are however in close contact, then liquids have a definite volume. Nevertheless, because the particles can move well-nigh each other rather freely, a liquid has no definite shape and takes a shape dictated by its container.
Liquids have the following characteristics:
- No definite shape (takes the shape of its container).
- Has definite volume.
- Particles are costless to move over each other, but are still attracted to each other.
A familiar liquid is mercury metallic. Mercury is an anomaly. It is the only metallic we know of that is liquid at room temperature. Mercury besides has an ability to stick to itself (surface tension)—a property that all liquids exhibit. Mercury has a relatively high surface tension, which makes information technology very unique. Hither you see mercury in its common liquid form.
Video \(\PageIndex{1}\): Mercury boiling to become a gas.
If we heat liquid mercury to its boiling point of \(357^\text{o} \text{C}\) under the right pressure conditions, we would find all particles in the liquid state become into the gas state.
Gases
If the particles of a substance have enough free energy to completely overcome intermolecular interactions, then the particles tin divide from each other and move about randomly in space. This describes the gas state, which we will consider in more detail elsewhere. Like liquids, gases take no definite shape, but different solids and liquids, gases accept no definite book either. The alter from solid to liquid usually does not significantly change the volume of a substance. Even so, the change from a liquid to a gas significantly increases the volume of a substance, by a factor of 1,000 or more than. Gases have the following characteristics:
- No definite shape (takes the shape of its container)
- No definite volume
- Particles motility in random motility with little or no attraction to each other
- Highly compressible
| Characteristics | Solids | Liquids | Gases |
|---|---|---|---|
| shape | definite | indefinite | indefinite |
| volume | definite | definite | indefinite |
| relative intermolecular interaction force | strong | moderate | weak |
| relative particle positions | in contact and fixed in place | in contact but not stock-still | non in contact, random positions |
What state or states of matter does each argument, describe?
- This state has a definite volume, but no definite shape.
- This state has no definite volume.
- This country allows the individual particles to move about while remaining in contact.
Solution
- This statement describes the liquid state.
- This statement describes the gas state.
- This argument describes the liquid country.
What country or states of thing does each statement describe?
- This state has individual particles in a stock-still position with regard to each other.
- This state has individual particles far autonomously from each other in infinite.
- This state has a definite shape.
- Answer a:
- solid
- Answer b:
- gas
- Respond c:
- solid
Summary
- Three states of matter exist—solid, liquid, and gas.
- Solids take a definite shape and volume.
- Liquids take a definite volume, only accept the shape of the container.
- Gases have no definite shape or volume.
Indefinite Shape And Definite Volume,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map:_Introductory_Chemistry_%28Tro%29/03:_Matter_and_Energy/3.03:_Classifying_Matter_According_to_Its_StateSolid_Liquid_and_Gas
Posted by: kleinmese1939.blogspot.com


0 Response to "Indefinite Shape And Definite Volume"
Post a Comment